Crystal structures and properties of mutagenic N-acyloxy-N-alkoxyamides — “most pyramidal” acyclic amides
Abstract
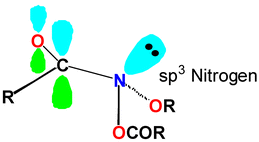
X-Ray data for two N-acyloxy-N-alkoxyamides, a class of direct-acting mutagens, indicate extreme pyramidalisation at the amide nitrogen in keeping with spectroscopic and theoretically determined properties of amides with bisoxo-substitution at nitrogen. The combined electronegativity of two oxygens leads to average angles at nitrogen of 107.8 and 108.1° and |χN| of 66° and 65°. The sp3 nature of nitrogen results in negligible amide resonance as evidenced by long N–C(O) bonds, high IR carbonyl stretch frequencies, carbonyl 13C NMR data and very low amide isomerisation barriers. In addition, conformations in the solid state support a strong nO–σ*NOAc anomeric interaction as predicted by molecular orbital theory. HF/6-31G* calculations on formamide, N-methoxyformamide and N-formyloxy-N-methoxyformamide support these findings.

 Please wait while we load your content...
Please wait while we load your content...