Competing sulfonylation and phosphonylation following rearrangement of an O-sulfonyl-N-phosphinoylhydroxylamine with tert-butylamine: demonstration of a phosphonamidic-sulfonic anhydride intermediate and 18O-labelling evidence on how it may be formed1
Abstract
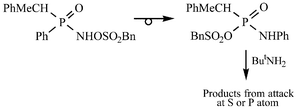
Reaction of R(Ph)P(O)NHOSO2Bn (R = PhMeCH) with ButNH2 in CH2Cl2 gives a mixture of RP(O)(NHPh)NHBut and ButNHSO2Bn, the proportion of the

 Please wait while we load your content...
Please wait while we load your content...