Investigation of the asymmetric ionic Diels–Alder reaction for the synthesis of cis-decalins
Abstract
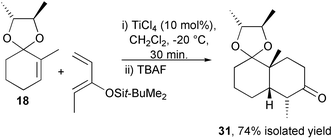
The ionic Diels–Alder reaction, whereby an α,β-unsaturated acetal in combination with a Lewis or Brønsted acid forms an equilibrium concentration of an activated dienophile, has been developed to provide an enantioselective synthesis of cis-decalins. Cyclohex-2-enone type chiral acetals of (2R,3R)-butane-2,3-diol have been screened against Lewis and Brønsted acids with a variety of dienes and are efficient for the synthesis of a limited subset of cis-decalin structures. Diastereoselectivities of 73% and 82% have been found for the asymmetric ionic Diels–Alder reaction between the chiral acetal derivatives of cyclohex-2-enone (6) and 2-methylcyclohex-2-enone (18) with 2,3-dimethyl-1,3-butadiene (7). Terminal substituents on the diene partner in general render the system unreactive. However a synthetically useful cis-decalin 31, derived from the reaction of 2-methylcyclohex-2-enone and Z-3-t-butyldimethylsilyloxypenta-1,3-diene has been prepared in enantiomerically pure form in 74% isolated yield using this asymmetric ionic Diels–Alder protocol.

 Please wait while we load your content...
Please wait while we load your content...