An alternative to the use of δ-lactam urethanes in the “ring switch” approach to higher homologues of AMPA-type glutamate antagonists
Abstract
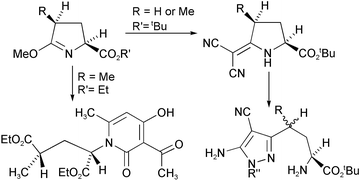
In an alternative strategy to the use of δ-lactam urethanes for the preparation of homologues of AMPA-type glutamate antagonists, we have used 5-exomethylene derivatives of pyroglutamate esters. The homochiral pyrazole amino acid derivatives 18 and 19 have been prepared in this way. Although this synthesis yields products with a glycine residue separated from a heterocyclic ring by two carbon atoms, the substitution of the heterocyclic ring is different from that in compounds prepared from δ-lactam urethanes. The branched chain compounds 32 and 33 have also been prepared in this way but the second chiral centre is epimerised during the synthesis. An interesting reaction, giving the pyridone 27 from the imino ether 24 and tert-butyl acetoacetate, is also reported.

 Please wait while we load your content...
Please wait while we load your content...