Application of the Barton photochemical reaction in the synthesis of 1-dethia-3-aza-1-carba-2-oxacephem: a novel agent against resistant pathogenic microorganisms
Abstract
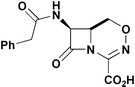
Racemic 7-(phenylacetamido)-1-dethia-3-aza-1-carba-2-oxacephem 3 was synthesized and found to possess antibacterial activity against Staphylococcus aureus FDA 209P, Escherichia coli ATCC 39188, Pseudomonas aeruginosa 1101–75 and Klebsiella pneumoniae NCTC 418 as well as the β-lactamase producing organisms E. coli A9675 and P. aeruginosa 18S-H and the methicillin-resistant organism S. aureus 95. Formation of the

 Please wait while we load your content...
Please wait while we load your content...