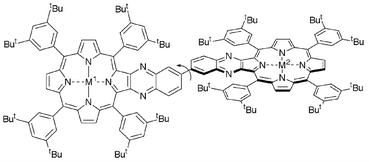
The synthesis of biquinoxalinyl-bridged bis-porphyrin 4 and metallated derivatives 5–11 was achieved in high yields. UV-visible spectroscopy and electrochemical experiments indicated weak orbital coupling of the two quinoxalinyl units but minimal orbital coupling between the two porphyrins. The weak electronic communication is attributed to non-planarity, on average, of the molecule because of rotation about the inter-quinoxalinyl connection. This, combined with poor coupling across the fused junctions between the porphyrin and quinoxalinyl units, results in minimal inter-porphyrin communication. As a result, the biquinoxalinyl linkage is appropriate for inclusion in more elaborate synthetic compounds, such as the tris-porphyrin 1, that are designed to model the charge-separation apparatus of Photosynthetic Reaction Centres.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...