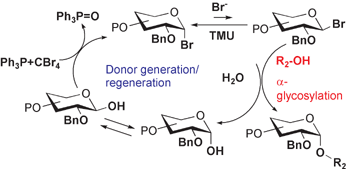
The reaction of a 2-O-benzyl-1-hydroxy sugar with CBr4 and Ph3P generates a glycosyl bromide in situ, which is coupled with an acceptor alcohol in the presence of N,N-tetramethylurea to afford an α-glycosyl product virtually quantitatively. In a proposed pathway, the reagent combination plays multiple roles such as the generation of a glycosyl donor, the activation of glycosylation, and the dehydration of the reaction system. These roles allow a simple α-glycosylation to be performed without special attention to dehydration. Various α-glycosyl (D-gluco-, D-galacto- and L-fuco-) products including glycosyl glycerols and cholesterols have been prepared with this method.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...