Smiles-type free radical rearrangement of aromatic sulfonates and sulfonamides: syntheses of arylethanols and arylethylamines
Abstract
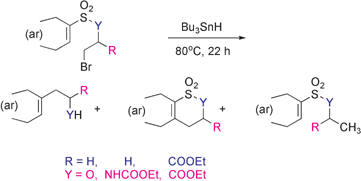
Smiles-type free radical rearrangements of arenesulfonates and arenesulfonamides are exploited for synthetic purposes. 4-Substituted

 Please wait while we load your content...
Please wait while we load your content...