Self-assembly of achiral and chiral macrocyclic ligands: synthesis, protonation constants, conformation and asymmetric catalysis
Abstract
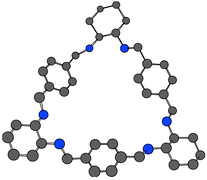
New 30-membered achiral and chiral polyaza macrocyclic ligands, L1 and L2 were synthesized directly from [3 + 3] condensation of phthalic dicarboxaldehyde with cis- and (1R,2R)-diaminocyclohexane, respectively. The trimeric macrocyclic structures were confirmed by electrospray ionization mass spectrometry (ESI-MS), 1H NMR, 13C NMR spectroscopy and elemental analysis. Potentiometry was used to determine the protonation constants of the ligands. UV–vis spectrophotometric titration was employed to investigate the coordination and conformational properties of the chiral ligand (L2). Direct enantioselective aldol reaction has been successfully performed using 4-nitrobenzaldehyde and acetone in the presence of the chiral macrocycle and its zinc(II) complexes as catalysts.

 Please wait while we load your content...
Please wait while we load your content...