Low-temperature X-ray structural studies of the ester and ether derivatives of cis- and trans-4-tert-butyl cyclohexanol and 2-adamantanol: application of the variable oxygen probe to determine the relative σ-donor ability of C–H and C–C bonds
Abstract
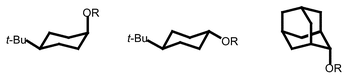
Results of low-temperature X-ray structural studies for five cis-, and three trans-4-tert-butyl cyclohexanol, and six 2-adamantanol ester and ether derivatives are reported. Plots of C–OR bond distance against pKa(ROH) for derivatives of axial alcohol (5), equatorial alcohol (6) and 2-adamantanol derivatives (7) give slopes of −2.77 × 10−3, −2.86 × 10−3 and −3.05 × 10−3, respectively. Given that the relative differences in the slopes are modest, no clear distinction can be made about the relative σ-donor ability of a C–H bond and a C–C bond.

 Please wait while we load your content...
Please wait while we load your content...