Stability against enzymatic hydrolysis of endomorphin-1 analogues containing β-proline
Abstract
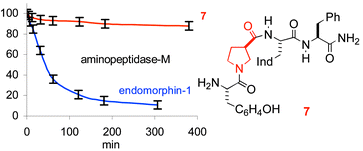
The enantiomer of endomorphin-1 (Tyr-Pro-Trp-PheNH2) and the analogues containing (S)- or (R)-β-proline have been synthesized, and their affinities towards μ-opioid receptors have been measured. As expected, the incubations of the different

 Please wait while we load your content...
Please wait while we load your content...