Synthesis of spirocycles viaring closing metathesis of heterocycles carrying gem-diallyl substituents obtained via ring opening of (halomethyl)cyclopropanes with allyltributyltin
Abstract
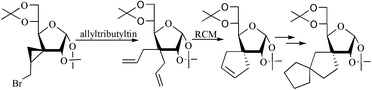
In the presence of allyl tri-n-butyltin–AIBN, cyclopropylmethyl bromides/xanthates undergo ring-opening reaction with concomitant formation of geminal diallyl derivatives in good yields. The ring closing metathesis reactions on geminal diallyl derivatives with Grubbs' catalyst provided spirocyclopentenyl products. Combination of these two methodologies has been applied to the synthesis of mono-, bis-cyclopentyl-carbohydrates as well as spirocyclopentylproline derivatives.

 Please wait while we load your content...
Please wait while we load your content...