6,19-Carbon-bridged steroids. Synthesis of 6,19-methanoprogesterone
Abstract
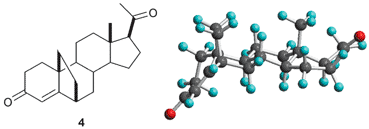
6,19-Methanoprogesterone (4) was synthesized in nine steps from the readily available 3β,20β-diacetyloxy-5α-bromo-6,19-oxidopregnane (5) in 14% overall yield. The additional carbon atom was introduced by reaction of a C-19

 Please wait while we load your content...
Please wait while we load your content...