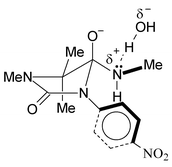
The pH-rate profiles for the cyclization of primary 2,3-dimethyl and 2,2,3-trimethyl-hydantoinamides (2-UAm and 3-UAm respectively) differ strikingly from those for the cyclizations of the corresponding N-methylated amides 2-MUAm and 3-MUAm; which are dominated by the water reaction, spanning some 6 pH units. For the cyclization of UAm the plateau extends over no more than two pH units. The difference is due to the slower base-catalyzed cyclization of the N-methylamides. The solvent kinetic isotope effect for this hydroxide-catalyzed reaction is close to 1.2, consistent with a slow protonation by water of the amino-group of the negatively charged tetrahedral intermediate. General base catalysis was observed with bases of pKBH+ up to 8. The Brønsted β are compatible with a hydrogen bonding mechanism for the GBC. In the gem-dimethyl compounds 3 the leaving group is flanked by substituents on both sides. The N-methyl group in 3-MUAm hinders frontal access of the proton, causing a 14000 fold decrease in rate. This is only 3800 fold in the compound with one methyl group at position 2.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...