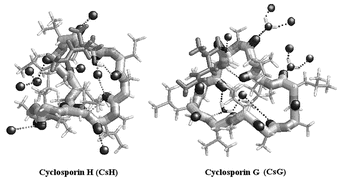
Cyclosporins are cyclic undecapeptides of fungal origin the best known of which, CsA, is a lead clinical immunosuppressant; CsG is a potential clinical immunosuppressant differing from CsA in residue 2 (L-α-aminobutyric acid in CsA, L-norvaline in CsG); and CsH is an inverse formyl peptide receptor agonist, differing from CsA in the chiral inversion of MeVal-11 from L to D. Crystal structure determinations of CsG and CsH were undertaken to identify structural and surface features important for biological activity and the future design of new cyclosporin derivatives. Ultra-high resolution X-ray structures (0.80 to 0.87 Å resolution) determined for two crystal forms of both CsH and CsG in the presence and absence of Mg2+ are described. A major outcome of this study is the observation that the local change in chirality between CsA and CsH is associated with a major structural transformation from open β-sheet in CsA to a highly convoluted conformation in CsH. CsG also possesses a completely novel cloverleaf motif with no H-bonded secondary structure features in spite of the minimal chemical difference with CsA. Unlike CsA, the structures of both CsH and CsG are heavily solvated. This study therefore shows that the chemical differences between the three cyclosporins, CsA, CsG and CsH can invoke unpredictably major differences in their 3D structures. The 9–11 cis-peptide bond in CsA moves to 11–1 in CsG, influencing the overall molecular conformation, while the peptide bonds in the highly convoluted loop conformation of CsH are all trans.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...