Syntheses and biological evaluation of vinblastine congeners†
Abstract
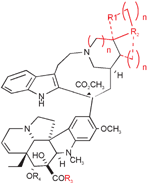
Sixty-two congeners of vinblastine (VLB), primarily with modifications of the piperidine ring in the carbomethoxycleavamine moiety of the binary alkaloid, were synthesized and evaluated for cytotoxicity against murine L1210 leukemia and RCC-2 rat colon cancer cells, and for their ability to inhibit polymerization of microtubular protein at <10−6 M, and for induction of spiralization of microtubular protein, and for microtubular disassembly at 10−4 M concentrations. An ID50 range of >107 M concentrations was found for L1210 inhibition by these compounds, with the most active 1000× as potent as vinblastine.

 Please wait while we load your content...
Please wait while we load your content...