Solvatochromism, halochromism, and preferential solvation of new dipolar guaiazulenyl 1,4-benzoquinone methides†
Abstract
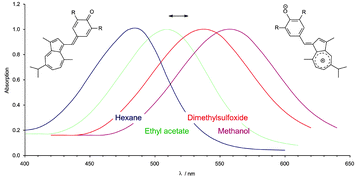
The synthesis and the solvatochromic properties of five dyes, obtained by condensation of guaiazulene with 4-hydroxybenzaldehydes, are described. Crystal structures of a quinoid dye and a phenolic dye precursor are presented. The dyes are sensitive to the dipolarity–polarizability of the medium and to the hydrogen-bond donor ability of protic solvents. Their solvatochromism is discussed in terms of Kamlet–Taft's π* and α scales, and their difference in behaviour is interpreted. Alkali and alkaline earth metal salts effect halochromism, with one exception due to extreme steric hindrance. Thus, this dye is capable of measuring solvent polarities without sensing the presence of electrolytes. Preferential solvation of the dyes in a series of binary solvent mixtures is explained quantitatively by solvent-exchange models.

 Please wait while we load your content...
Please wait while we load your content...