From central to planar chirality, the first example of atropenantioselective cycloetherification
Abstract
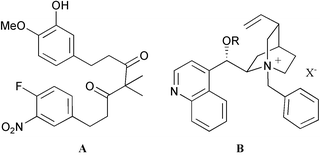
Using chiral quaternary ammonium hydroxide as base, cycloetherification of linear achiral diarylheptanoid 5, by way of an intramolecular SNAr reaction, provides enantiomerically enriched cyclophane 6 in good to excellent yield.

 Please wait while we load your content...
Please wait while we load your content...