A stereodivergent, two-directional synthesis of stereoisomeric C-linked disaccharide mimetics
Abstract
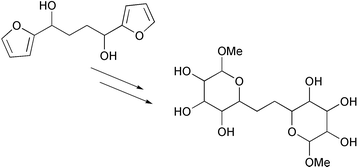
Dipyranones, such as 1,2-bis[(2R,3S,6S)-3-hydroxy-6-methoxy-3-oxo-6H-pyran-2-yl]ethane, were exploited as templates for the synthesis of some novel C-linked disaccharide analogues. Efficient methods, such as stereoselective reduction and dihydroxylation, were developed for two-directional functionalisation of these templates. Peracetylated derivatives of ten stereoisomeric disaccharide analogues {acetic acid 4,5-diacetoxy-6-methoxy-[(3′,4′,5′-triacetoxy-6′-methoxytetrahydropyran-2′-yl)ethyl]tetrahydropyran-3-yl esters} were synthesised from a virtual library of 136 compounds; furthermore, an additional eight stereoisomers could have been synthesised simply by using the enantiomeric ligand in the enantioselective step. The ability of (2S,3S,4R,5R,6R)- 6-methoxy-2-[2′-((2′R,3′R,4′S,5′R,6′S)-3′,4′,5′-trihydroxy-6′-methoxytetrahydropyran-2′-yl)ethyl]tetrahydropyran-3,4,5-triol to bind to the repressor protein, LacI, was estimated to be similar to that of isopropyl-β-thiogalactoside. The disaccharide mimetics were concluded to be a new and interesting class of C-linked disaccharide mimetics with promising, though largely unstudied, biological activity.

 Please wait while we load your content...
Please wait while we load your content...