Bisubstrate inhibitors for the enzyme catechol-O-methyltransferase (COMT): influence of inhibitor preorganisation and linker length between the two substrate moieties on binding affinity
Abstract
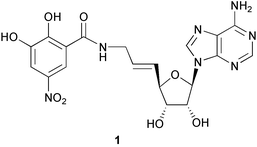
Inhibition of the enzyme catechol-O-methyltransferase (COMT) is an important approach in the treatment of Parkinson's disease. A series of new potent bisubstrate

 Please wait while we load your content...
Please wait while we load your content...