Non-steady-state kinetic study of the SN2 reaction between p-nitrophenoxide ion and methyl iodide in aprotic solvents containing water. Evidence for a 2-step mechanism
Abstract
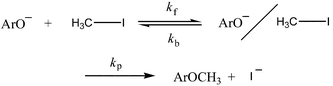
Non-steady-state kinetic studies reveal that the SN2 reaction between p-nitrophenoxide ion and

 Please wait while we load your content...
Please wait while we load your content...