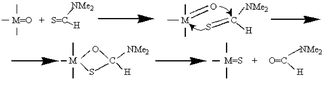
Molybdenum and tungsten oxoalkoxides {[MO(OMe)4]; M = Mo, W} and rhenium heptaoxide (Re2O7) reacted at room temperature with thiocarbonyl compounds such as N,N-dimethylthioformamide (DMTF), producing the crystalline metal sulfides MoS2, WS3 and Re2S7, respectively. The reaction mechanism involves, as the first step, the coordination of DMTF to a metal via the sulfur donor atom, followed by the subsequent metathesis of the latter with the doubly bonded oxygen atom. The final product is, depending on reaction conditions, a colloidal metal sulfide or up to 0.1 mm large metal sulfide crystals. By carrying out the reaction within a mesoporous alumina matrix, supported metal sulfide catalysts were obtained in one step. These catalysts were tested for catalytic hydrodesulfurization and their activity compared with catalysts prepared by traditional methods. Reaction of nickel and zinc acetylacetonates and aminoalkoxides with DMTF in hydrocarbon media was found to provide colloids and, on aging, fine powder precipitates of NiS and ZnS.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...