Mechanistic inferences of the photocatalyzed oxidation of chlorinated phenoxyacetic acids by electrospray mass spectral techniques and from calculated point charges and electron densities on all atoms
Abstract
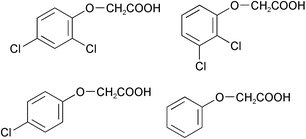
The photodegradation of 2,4-dichlorophenoxyacetic acid (2,4-D) taking place in UV irradiated aqueous TiO2 dispersions was revisited to obtain mechanistic details on the basis of similar degradation of the related 2,3-dichlorophenoxyacetic acid (2,3-D), 4-chlorophenoxyacetic acid (4-M) and phenoxyacetic acid (PhA). Mechanistic changes were inferred from the different positions of the chlorine substituents. As well, the compounds were compared for differences in degradation rate and initial adsorption on the TiO2 surface as a function of the number of chlorines. The initial mechanistic sequence(s) in the TiO2-photocatalyzed oxidation of each substrate was predicted theoretically by molecular orbital (MO) calculations of frontier electron densities and point charges of all the atoms in the phenoxyacetic acid structures.

 Please wait while we load your content...
Please wait while we load your content...