Synthesis and the electrochemical and fluorescence properties of new cyclophane-derivatized oligothiophenes
Abstract
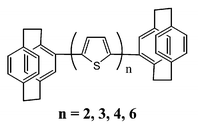
Several new oligothiophenes blocked by cyclophane end groups with 2, 3, 4 or 6 thiophene rings in the chain have been prepared and studied by electrochemistry and by UV-visible and fluorescence spectroscopy. Both radical cations and dications are stable, even for the bithiophene compound; besides the stabilization induced by the blocking of the reactive α positions, this strongly suggests electronic connection between the cyclophanes and the oligothiophene chains, especially for the shorter compounds. All the molecules are strongly fluorescent with increasing quantum yields up to 4 thiophene rings. The assumption of a conjugation between the cyclophane end groups and the oligothiophene main chain is also supported by the UV-visible and fluorescence results, since the spectroscopic data correlate well with (n + 2)−1 instead of n−1, with n representing the number of thiophene rings in the oligomer.

 Please wait while we load your content...
Please wait while we load your content...