C–H bond activation reactions by TpMe2Ir(iii) centres. Generation of Fischer-type carbenes and development of a catalytic system for H/D exchange†
Abstract
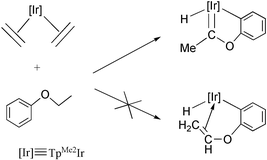
The unsaturated [TpMe2Ir(C6H5)2] fragment, readily generated from [TpMe2Ir(C6H5)2(N2)], or from [TpMe2Ir(C2H4)2] and C6H6, is able to induce the regioselective cleavage of two sp3 C–H bonds of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Me)C(Me)
C(Me)C(Me)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)], accomplishes the efficient, catalytic H/D exchange between C6D6
(used as the deuterium source) and a variety of organic and organometallic molecules that contain C–H bonds of different nature.
CH2)], accomplishes the efficient, catalytic H/D exchange between C6D6
(used as the deuterium source) and a variety of organic and organometallic molecules that contain C–H bonds of different nature.

 Please wait while we load your content...
Please wait while we load your content...