Synthesis, redox behaviour and X-ray structure of bis-TTF containing a pyridine unit as a potential building block in the construction of conducting magnetic materials
Abstract
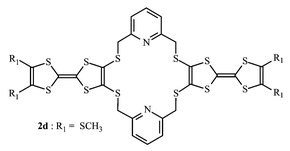
The synthesis of singly and doubly bridged bis-TTF containing a 2,6-bis(methylthio)pyridine group as a linker and potentially usable as a

 Please wait while we load your content...
Please wait while we load your content...