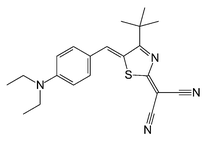
The solvatochromism of the novel dye 4-tert-butyl-2-(dicyanomethylene)-5-[4-(diethylamino)benzylidene]-Δ3-thiazoline (1) has been investigated in 26 solvents of different polarity. 1 exhibits a positive solvatochromism, its solvent-induced bathochromic UV/Vis absorption band shift ranges from n-hexane (λmax = 566 nm) to dimethyl sulfoxide (λmax = 640 nm). The molar absorption energy of the solvatochromic band shift of 1 can be well correlated with Kamlet–Taft's and Catalàn's solvent polarity scales. 1 is mainly sensitive to the solvent's dipolarity/polarizability (π* of SPPN term) rather than of its HBD (hydrogen-bond donating) or HBA (hydrogen-bond accepting) property. It is emphasized that the UV/Vis absorption band maximum of 1 in the strong HBD solvents acetic acid, 2,2,2-trifluoroethanol and 1,1,1,3,3,3-hexafluoropropan-2-ol fit well in the LSE (linear solvation energy) relationship with Kamlet and Taft's π* and Catalàn's SPPN scale, respectively, which makes this dye important as a dipolarity/polarizability indicator for various solid acids.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...