Chemoenzymatic total syntheses of the sesquiterpene (−)-patchoulenone†
Abstract
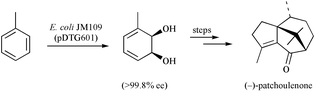
The bicyclo[5.3.1]undec-7(8)-en-3-one 6, which is prepared from the monochiral cis-1,2-dihydrocatechol 4, affords a mixture of products 7, 8 and 9 on exposure to protic acid. Each of compounds 8 and 9 rearranges to congener 7 on treatment with SnCl2 or upon sustained reaction with protic acid. Reaction of the last compound with hydrogen in the presence of palladium on carbon affords a mixture of the saturated diols 10 and 11 with the latter capable of elaboration to (−)-patchoulenone (1) in four simple steps. An alternate and more efficient route to compound 11 involved a radical cyclisation route wherein enone 6 was treated with SmI2 and thiophenol, the latter reagent being employed to ensure efficient reduction. The major product, 12, thus formed was then debenzylated to give the patchoulenone precursor 11. In an even more efficient route to the title sesquiterpene, the bicyclo[2.2.2]octenone 21 was reacted with isopropenyllithium to give the dienol 22, which engaged in an anionic oxy-Cope rearrangement to afford the bicyclo[5.3.1]undec-7(8)-enone 23 and for which an X-ray crystal structure determination has been carried out. Reductive cyclisation of the last compound using SmI2 in the presence of thiophenol then gave, in a stereoselective manner, diol monoether 24, which after subjection to debenzylation, oxidation and dehydration steps afforded (−)-patchoulenone (1).

 Please wait while we load your content...
Please wait while we load your content...