Origin of the mediator losses in electrochemical delignification processes: primary and secondary reactions of violuric acid and N,N′-dimethylvioluric acid radicals with lignin model compounds
Abstract
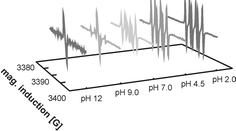
The use of mediator-based processes for the delignification of wood pulps is of great interest as they promise to be environmentally as well as economically interesting alternatives to the currently dominating chlorine-based processes. Mediator-based processes suffer from the fact that substantial amounts of the mediator molecules are irreversibly lost during the reactions. We have now analyzed in detail the primary and in addition the secondary reactions of the

 Please wait while we load your content...
Please wait while we load your content...