Neoteric solvents for asymmetric hydrogenation: supercritical fluids, ionic liquids, and expanded ionic liquids†
Abstract
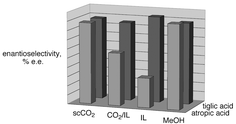
Neoteric (new) solvents such as supercritical CO2 (scCO2), ionic liquids (ILs), ILs with cosolvents, and CO2-expanded ionic liquids (EILs) offer flexible physical properties, which allow chemists and engineers to select the optimal solvent system for a specific reaction process. Homogeneously-catalyzed asymmetric hydrogenation of α,β-unsaturated carboxylic acids was chosen for its economic interest and its multiple H2-concentration dependent behaviours. For example, with ruthenium BINAP-type catalysts, type I substrates require high H2 concentration in solution, while type II require low H2 concentration. ScCO2, ILs and EILs are highly attractive because of their contrasting properties and their potential flexibility in improving or reducing hydrogen transfer rates and thus concentrations. Several ILs were tested and compared with EILs, IL–cosolvent mixtures, scCO2, and normal methanol as media for these reactions to establish the most effective system for each substrate type. Atropic acid (type I) was hydrogenated up to 92% ee which is not better than in methanol. However, tiglic acid (type II) was hydrogenated up to 93% ee in the optimized IL system, which is significantly better than was observed in MeOH. CO2-expansion of ionic liquids affected the selectivity for both substrates, improving the selectivity for atropic acid and lowering it for tiglic acid. The solubility of the catalyst in scCO2 was measured and the antisolvent effect of H2 in scCO2 was demonstrated and discussed.

 Please wait while we load your content...
Please wait while we load your content...