Zinc dicarboxylate polymers and dimers: thiourea substitution as a tool in supramolecular synthesis†
Abstract
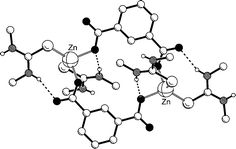
The reactions of the zinc thiourea complexes [Zn{SC(NHR)(NHR′)}4]Cl2
(L =
L1, R = H, R′
= Me; L =
L2, R = Me, R′
= Me) with a range of dicarboxylates have been investigated. From these reactions eleven products –
[Zn(L1)2(μ-terephthalate)]n, [Zn(L1)2(μ-fumarate)]n, {[Zn(L1)2(μ-isophthalate)]·H2O}n, [Zn(L1)2(μ-1,3-phenylenediacetate)]n, [Zn(L1)2(μ-phthalate)]2·4H2O, {[Zn(L2)2(μ-terephthlate)]·0.5H2O}n, [Zn(L2)2(μ-fumarate)]n, [Zn(L2)2(μ-isophthalate)]2·2H2O, [Zn(L2)2(μ-phthalate)]n, [Zn(L2)2(μ-maleate)]2·2H2O and [Zn(L2)2(μ-citraconate)]2·2H2O – have been crystallographically characterised. The structural characterisation of these compounds demonstrates that by increasing the number of methyl substituents on the

 Please wait while we load your content...
Please wait while we load your content...