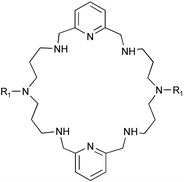
Two 28-membered octaazamacrocycles, [28]py2N6 and Me2[28]py2N6, have been synthesized. The protonation constants of the N-methyl derivative and the stability constants of its complexes with Ni2+, Cu2+, Zn2+, Cd2+, and Pb2+ were determined at 25 °C in 0.10 mol dm−3 KNO3. The high overall basicity of Me2[28]py2N6 is ascribed to the weaker repulsion between protonated contiguous charged ammonium sites separated by propyl chains. These studies together with NMR, UV-vis and EPR spectroscopies indicated the presence of mono- and di-nuclear species. The single crystal structure of the complex [Ni2([28]py2N6)(H2O)4]Cl4·3H2O was determined, and showed each nickel centre in a distorted octahedral co-ordination environment. The nickel centres are held within the macrocycle at a large distance of 6.991(8)
Å from each other. The formation of mononuclear complexes was evaluated theoretically via molecular mechanics (MM) and molecular dynamics (MD) calculations and showed that these large macrocycles have sufficient flexibility to encapsulate metal ions with different stereo-electronic sizes. Structures for small and large metal ions are proposed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...