The cyclotriphosphate ion (P3O93−) as a PPN [PPN =
(Ph3P)2N+] salt reacted in CH2Cl2 at room temperature with the cationic solvated complexes of Pd(II) and Pt(II), [M(phosphine)2(Me2CO)2]2+
[M = Pd, Pt; phosphine = PPh3, PMePh2, 1/2 Ph2P(CH2)2PPh2
(dppe), 1/2 Ph2P(CH2)4PPh2
(dppb)], to give the anionic P3O9 complexes (PPN)[Pd(P3O9)(PPh3)2]
(1), (PPN)[Pd(P3O9)(PMePh2)2]
(2), (PPN)[Pt(P3O9)(PPh3)2]
(3), (PPN)[Pt(P3O9)(PMePh2)2]
(4), (PPN)[Pt(P3O9)(dppe)]
(5) and (PPN)[Pt(P3O9)(dppb)]
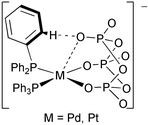
(6). Crystallographic studies revealed that the P3O9 ligand in complexes 1–4 and 6 adopts a normal chair conformation and behaves as a pseudo-tridentate ligand with two normal M–O bonds and an additional weak M⋯O interaction. In 1 and 3, the terminal P3O9 oxygen atom weakly bound to the metal centre forms relatively strong intramolecular CH⋯O hydrogen bonds with the phosphine ligands. In contrast, the P3O9 ligand in 5 is bidentate and takes a pseudo-boat conformation. Complexes 1–6 are fluxional in solution and exhibit only one singlet due to the P3O9 ligand in the 31P–{1H} NMR spectra at room temperature; the signals of complexes 4–6 split into two at −40 to −70 °C. The activation parameters for the fluxional behaviour of 6 were determined by the line shape analysis of the variable temperature 31P–{1H} NMR spectra. The Pt(IV) complex (PPN)2[PtMe3(P3O9)]
(7) was also synthesised and structurally characterised.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...