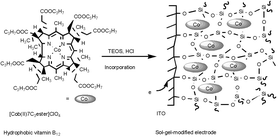
A vitamin B12 derivative, heptapropyl cobyrinate perchlorate, was readily trapped onto an indium tin oxide (ITO) electrode by a sol–gel reaction. The complex was physically retained in a silica gel film which is formed on an ITO electrode. The thickness of the film could be controlled by the withdrawing speed of the dip coating process. Formation of a sol–gel film was confirmed by SEM measurements, and the total amount of the complex in the film was determined by UV–VIS absorption spectra. The complex exhibits the CoII/CoI redox couple at −0.42 V vs. Ag–AgCl. The amount of the electroactive complex in the sol–gel film deduced from electrochemical measurements is 3.0 × 10−11 and 6.2 × 10−11 mol cm−2 for thicknesses of 170 and 330 nm, respectively. This electroactive complex shows a high reactivity towards organic halides, and the controlled-potential electrolysis of benzyl bromide using the sol–gel modified electrode at −1.20 V vs. Ag–AgCl in aqueous solution containing 0.1 M KCl afforded dehalogenated products, bibenzyl and toluene, with a total turnover number of >1000 for 1 h.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...