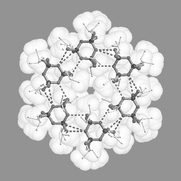
Herein, we introduce an extremely ‘soft’ tecton, which interacts via
‘soft’ synthons displaying an unprecedented variety of supramolecular architectures in the solid state. Hexakis(organoamino) cyclotriphosphazene derivatives, (RNH)6P3N3, contain a polar core comprising an equatorial belt of three ring nitrogen atoms and six NH functions which is sandwiched between hemispheres of lipophilic substituents R. A range of derivatives equipped with hydrocarbon side chains were synthesised and structurally characterised including R =
tert-butyl (1), cyclohexyl (2), iso-propyl (3), benzyl (4), 2-phenylethyl (5), iso-butyl (6), phenyl (7), p-tolyl (8), n-propyl (9), allyl (10), propargyl (11) and methyl (12). The study shows that subtle modifications of the lipophilic periphery lead to considerable changes in the solid-state aggregation pattern. With the exception of 1 all solid-state structures show intermolecular NH⋯N bonding with motifs containing one, two, three and four H-bridges. Supramolecular architectures include monomer (1), dimer (2), cyclic hexamer (3), zigzag chain (4, 6), linear chain (5·thf, 7, 8), double chain (9), graphite-type sheet (10), rectangular grid (11) and hexagonal close-packed sheet (12). The structural variety is due to easy rotation around exocyclic P–N bonds, which allows variable directionalities of all six N–H bonds. M.O. calculations on the gas phase dimer of (H2N)6P3N3 mirror the H-bridging motifs observed in crystal structures of (RNH)6P3N3 derivatives.

 Please wait while we load your content...
Please wait while we load your content...