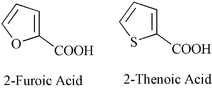
Complexation of thorium(iv) with 2-furoic acid and 2-thenoic acid in aqueous solution
Abstract
Complexation of Th(IV) with  −
− j)+, where L stands for the furoate or thenoate anion and j = 1–3 for
j)+, where L stands for the furoate or thenoate anion and j = 1–3 for  −
− j)+ complexes, in contrast to earlier results in the literature which suggest that the protonated complexes ThL4H22+ and ThL3H23+ are dominant under the experimental conditions. The binding strength of
j)+ complexes, in contrast to earlier results in the literature which suggest that the protonated complexes ThL4H22+ and ThL3H23+ are dominant under the experimental conditions. The binding strength of

 Please wait while we load your content...
Please wait while we load your content...