Cyclometallated imine platinum(IV) derivatives of general formula [PtX(Me)2(C–N)(SMe2)], being X = Br and Cl with C–N = CC5H4CH2NCH(2,4,6-Me3C6H2)
(1 and 2); X = I with C–N = CC5H4CHNBzl (3); X = Br with C–N = CC5H4CHNPh and CC5H4CH2N(2,4,6-Me3C6H2)
(4 and 5), and X = I with C–N = CC5H4CH2N(2,4,6-Me3C6H2)
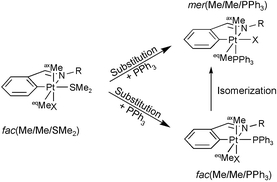
(6) have been characterised as having different fac(Me/Me/SMe2) to mer(Me/Me/SMe2) geometrical disposition ratios. The isomeric fac(Me/Me/SMe2) form is present at various percentages depending on the rigidity of the system, from a maximum of 100% for complexes 1 and 2 as proved from the X-ray crystal structure determination of both complexes and selective NOE experiments, to a minimum of 30% in the case of 3. SMe2 by PPh3 substitution has been studied for these fac complexes where, in comparison to similar compounds with a mer(Me/Me/SMe2) disposition, the strong trans-influence of the methyl group is avoided. The results indicate that there are more important effects than the simple trans-influence of the methyl ligand, the reactions are found to be much faster than their equivalent with complexes with mer geometries. In some of the cases, the final phosphine substituted products show the full isomerization to expected less hindered mer(Me/Me/PPh3) geometry, while in others, a mixture of the fac and mer arrangements is found in the final reaction products. For the 2,4,6Me3C6H2-containing imines the isomerization takes place during substitution from a postulated pentacoordinated intermediate, for the remaining complexes the final reaction mixture is obtained in a process that takes place after substitution. For these two cases (products 3 and 4), the presence of an intermediate with a fac(Me/Me/PPh3) geometry, that further evolves to the final mer complex, has been characterised by low temperature NMR, and the isomerization reactions have been studied as a function of temperature and pressure. The results agree with a process involving a very energetically demanding turnstile twist type reorganization of the molecule, after a significant degree of dissociation of the ligands (ΔH‡
= 109 and 100 kJ mol−1; ΔS‡
= 88 and 49 J K−1 mol−1; ΔV‡
= 14.9 and 20.0 cm3 mol−1 for compounds 3 and 4 respectively).

 Please wait while we load your content...
Please wait while we load your content...