Heat capacity of SrFeO3−δ; δ = 0.50, 0.25 and 0.15 – configurational entropy of structural entities in grossly non-stoichiometric oxides†
Abstract
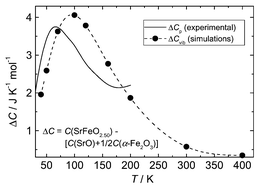
The heat capacity of SrFeO3−δ; δ
= 0.50, 0.25 and 0.15 has been determined for 10 < T/K < ≈ 800 K by adiabatic

 Please wait while we load your content...
Please wait while we load your content...