Competitive electron transfers from a tyrosyl side-chain and peptide bond in the photodegradation of N-tosyl α-aminomethylamides: an insight into photosynthesis and photodamage in the biological oxidation of water?
Abstract
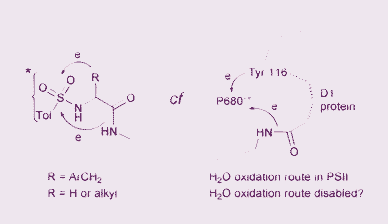
Photo-excited N-tosyl derivatives of

 Please wait while we load your content...
Please wait while we load your content...