Stereoselective aza-Diels–Alder reactions with 2H-azirines as dienophiles furnishing highly functionalized tetrahydropyridines
Abstract
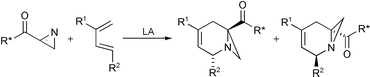
Highly diastereoselective Lewis acid mediated aza-Diels–Alder reactions of chiral auxiliary derivatized 2H-azirines have been accomplished for the first time, yielding bi and tri-cyclic

 Please wait while we load your content...
Please wait while we load your content...