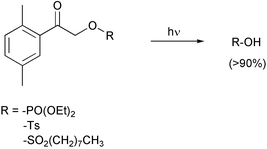
2,5-Dimethylphenacyl esters: A photoremovable protecting group for phosphates and sulfonic acids†
Abstract
2,5-Dimethylphenacyl phosphoric and sulfonic esters release the corresponding acids upon irradiation in nearly quantitative isolated yields, with quantum yields Φ
= 0.71 and 0.68 in

 Please wait while we load your content...
Please wait while we load your content...