Photoredox, photodecarboxylation, and photo-retro-Aldol chemistry of p-nitrobiphenyls
Abstract
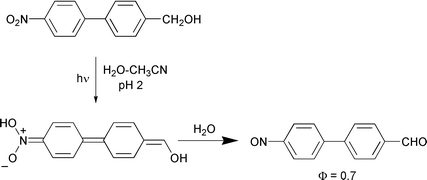
The photochemistry of three p-nitrobiphenyl derivatives 9–11 has been investigated to explore the ability of photoexcited nitro groups to induce chemistry through the biphenyl ring system. Previous work has shown that the nitro group is highly electron withdrawing at both the meta and para positions (on the benzene ring) in the excited triplet state, inducing decarboxylations and retro-Aldol type reactions, as well as a novel intramolecular redox-type reaction. The mechanisms of all of these reactions are believed to involve photogenerated nitrobenzyl carbanion-type intermediates. Analogous reactions with enhanced quantum efficiencies were observed in the nitrobiphenyls studied in this work. This is further evidence that twisted ground state biaryls (and possibly higher order oligophenylenes) may be thought of as highly polarizable electronically conjugated π-systems in the excited state with the ability to induce efficient photochemistry. Moreover, a charge transfer triplet state is believed to be responsible for inducing highly efficient, novel acid-catalyzed pathways observed for the photodecarboxylation of 11 and the photoredox reaction of 9, neither of which has been observed in the corresponding nitrophenyl (parent) system.

 Please wait while we load your content...
Please wait while we load your content...