Ring closure synthetic strategies toward buckybowls: benzannulation versus cyclopentannulation†
Abstract
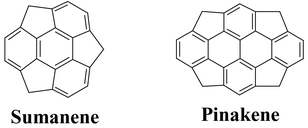
Computations were performed on idealized retrosynthetic routes towards two model buckybowl compunds, sumanene (I) and pinakene (II). Two possible paths for sumanene (I) and six for pinakene (II) were analyzed. The computational results unequivocally predict that benzannulation is a significantly easier process compared to cyclopentannulation in the ring closure strategies in both cases. The suitability of the theoretical models for obtaining reliable trends is assessed and generalizations for the synthetic strategies directed towards buckybowls and C60 were made.

 Please wait while we load your content...
Please wait while we load your content...