Intramolecular nucleophilic catalysis and the exceptional reactivity of N-benzyloxycarbonyl α-aminophosphonochloridates
Abstract
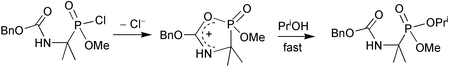
The chloridate BnOCONHCH2P(O)(OMe)Cl is 103–104 times more reactive than ClCH2P(O)(OMe)Cl in substitution with PriOH; the α,α-dimethyl analogue is no less reactive and the N-methyl derivative is more reactive; nucleophilic participation (catalysis) by the carbamate group is implicated.

 Please wait while we load your content...
Please wait while we load your content...