Kinetics and mechanism of the oxidation of ethyl xanthate and ethyl thiocarbonate by hydrogen peroxide
Abstract
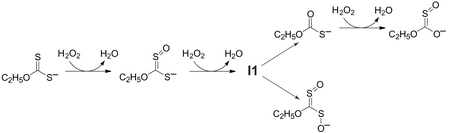
Kinetic studies on the oxidation of ethyl xanthate (O-ethyl dithiocarbonate) by hydrogen peroxide have been conducted for a range of ethyl xanthate and hydrogen peroxide concentrations over the pH range 8–12. The initial reaction product is a canonical form of O-ethyl S-oxodithiocarbonate. Further oxidation leads to a bifurcation in the reaction pathway, with the formation of either O-ethyl thiocarbonate or a canonical form of O-ethyl S-oxoperoxydithiocarbonate. The partitioning of the reaction between these alternative reaction paths is pH dependent, with the proportion directed towards the O-ethyl thiocarbonate branch increasing over the pH range 10 to12. Further oxidation of O-ethyl thiocarbonate leads to the formation of O-ethyl S-oxothiocarbonate (or a canonical form thereof), analogous to the initial oxygen addition to ethyl xanthate. For both reaction branches the ultimate sulfur-containing product is sulfate. Apart from the process controlling the bifurcation, the reaction kinetics can be modelled as a series of bimolecular oxygen addition steps. This kinetic model is supported by hydroxyl radical scavenging experiments (using tert-butyl alcohol) that suggest no involvement by OH˙. The pH dependence of the rate parameters indicates that reaction occurs exclusively with H2O2 rather than HO2−, consistent with the expected nucleophilic attack at the peroxide oxygen. The process controlling the partitioning between the two alternative pathways is proposed to originate from an oxygen addition adduct of O-ethyl S-oxodithiocarbonate. This work reveals that a range of potential metal ion complexants may be produced in the industrial application of xanthates (primarily sulfide mineral extraction, but also including viscose rayon production and pesticide manufacture), and that the environmental chemistry of these reagents is more complex than has been previously appreciated.

 Please wait while we load your content...
Please wait while we load your content...