Twisting and planarization in push–pull ethylenes†
Abstract
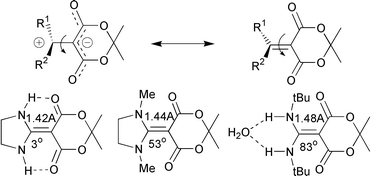
As determined by ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds between 3 and 83°. Hydrogen bonds between substituents RHN and the carbonyl groups favour near-planarity. Sterically demanding substituents favour large dihedral angles and zwitterionic structures as in formula 20. AM1 calculations of the structures are in excellent agreement with the experimental X-ray data, provided a dielectric field is incorporated (ε
= 40). This can be ascribed to the highly polar (zwitterionic) nature of the molecules. It is further predicted that all these molecules, including those that are stabilised in a planar form by intramolecular hydrogen bonds, undergo rapid rotation about the central C
C double bonds between 3 and 83°. Hydrogen bonds between substituents RHN and the carbonyl groups favour near-planarity. Sterically demanding substituents favour large dihedral angles and zwitterionic structures as in formula 20. AM1 calculations of the structures are in excellent agreement with the experimental X-ray data, provided a dielectric field is incorporated (ε
= 40). This can be ascribed to the highly polar (zwitterionic) nature of the molecules. It is further predicted that all these molecules, including those that are stabilised in a planar form by intramolecular hydrogen bonds, undergo rapid rotation about the central C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds at room temperature.
DFT calculations incorporating a dielectric field model (
C bonds at room temperature.
DFT calculations incorporating a dielectric field model (

 Please wait while we load your content...
Please wait while we load your content...