The concept of the real stability constant for complexes formed through hydrogen bonding
Abstract
On the basis of previously published results it is explained why the formation constants of complexes formed through hydrogen bonding, contrary to accepted opinion (e.g. Shan et al., Science, 1996, 272, 97), are not the general measure of their stability. Instead, a newly introduced quantity is recommended—the real stability constant—which takes into account all possible ways of decomposition of such complexes. The practical expression for the real stability constant of AHA−1 type complexes (formed in reactions between HA and A−1 with comparable steric contributions) exhibits a distinct maximum at ΔpKSa (defined as pKSHA1 − pKSHA) equal to or slightly different from zero depending on whether the hydrogen bridges join moieties belonging to compounds of the same or different families. The two major factors governing the value of this constant are the comparable proton donating properties of HA and HA1 and the tendency towards homoconjugation in the parent systems HA + A− and HA1 + A−1. Thus, a problem which for years seemed intuitively understandable, has finally found both theoretical explanation and experimental confirmation. The general solution found in the paper creates a base for much more constructive discussion on the proposal of Cleland and Kreevoy (Science, 1994, 264, 1887) dealing with enzymatic catalysis.
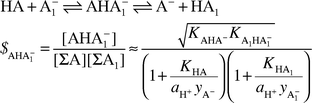

 Please wait while we load your content...
Please wait while we load your content...