Dilute mixtures of n-heptanal in synthetic air (up to 100 ppm) were photolyzed with fluorescent UV lamps (275–380 nm) at 298 K. The main photooxidation products, identified and quantitatively analyzed by FTIR spectroscopy, were pent-1-ene, CO, vinyl alcohol and ethanal. The photolysis rates and the absolute quantum yields Φ were found to be slightly dependent on the total pressure. At 100 Torr, Φ100
= 0.36 ± 0.03, whereas at 700 Torr the total quantum yield was Φ700
= 0.31 ± 0.01. The results may be explained by the collisional deactivation of photoexcited molecules. Two decomposition channels were identified: the radical channel C6H13CHO → C6H13
+ HCO, and the molecular channel C6H13CHO → C5H10
+ CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHOH, having the absolute quantum yields of 0.031
and 0.118 at 700 Torr. The product CH2
CHOH, having the absolute quantum yields of 0.031
and 0.118 at 700 Torr. The product CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHOH tautomerizes to ethanal.
CHOH tautomerizes to ethanal.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHOH, having the absolute quantum yields of 0.031
and 0.118 at 700 Torr. The product CH2
CHOH, having the absolute quantum yields of 0.031
and 0.118 at 700 Torr. The product CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHOH tautomerizes to
CHOH tautomerizes to 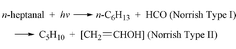

 Please wait while we load your content...
Please wait while we load your content...