Hydrosilylation of cinchonidine and 9-O-TMS-cinchonidine with triethoxysilane: application of 11-(triethoxysilyl)-10,11-dihydrocinchonidine as a chiral modifier in the enantioselective hydrogenation of 1-phenylpropane-1,2-dione
Abstract
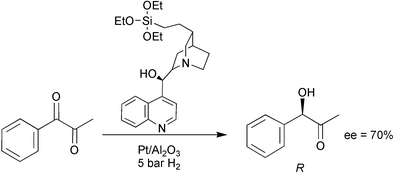
The detailed synthesis and characterization of (−)-11-(triethoxysilyl)-10,11-dihydrocinchonidine (4), a starting material for the immobilization of (−)-cinchonidine on silica based supports, is described. Compound 4 together with its precursors 9-O-(trimethylsilyl)cinchonidine (2) and 9-O-(trimethylsilyl)-11-(triethoxysilyl)-10,11-dihydrocinchonidine (3) were employed as chiral modifiers in the hydrogenation of a prochiral diketone, 1-phenylpropane-1,2-dione, over a heterogeneous Pt/Al2O3 catalyst using cinchonidine (1) as a reference modifier. The unexpected enhancement of ee induced by 4, demonstrating the positive effect of distal modifier substitution, is discussed in the light of molecular modeling and NMR studies.

 Please wait while we load your content...
Please wait while we load your content...