Reaction of 5-halo-1,2,3-thiadiazoles with arylenediamines as a new approach to tricyclic 1,3,6-thiadiazepines
Abstract
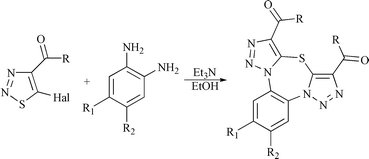
A multistep reaction of 5-halo-1,2,3-thiadiazoles and 1,2-phenylenediamines provides a new route to fused 1,3,6-thiadiazepines. The overall process consists of the known stepwise formation of

 Please wait while we load your content...
Please wait while we load your content...